When a rheumatologist prescribes Humira instead of a biosimilar, or an oncologist chooses Ocrevus over a cheaper alternative, it’s not because they’re ignoring cost-it’s because they’ve seen what happens when patients switch.
Specialty drugs aren’t like your typical pills you pick up at the corner pharmacy. These are high-cost, complex medications used for serious, often rare conditions: multiple sclerosis, rheumatoid arthritis, cancer, Crohn’s disease. They’re injected or infused. They need special storage. They come with strict monitoring requirements. And even though they make up less than 7% of all prescriptions, they account for more than 70% of total drug spending in the U.S.
So why do specialists keep reaching for the brand-name version-even when generics or biosimilars are available? It’s not about loyalty to a company. It’s about safety, predictability, and real-world outcomes.
They’ve Seen the Consequences of Switching
One of the most common reasons specialists avoid switching patients to biosimilars or generics is personal experience. A 2023 Medscape survey found that 68% of specialists feel frustrated by prior authorization delays-but even more, they’ve seen patients flare up after a switch.
Take the case of a patient with moderate to severe psoriasis. Their dermatologist had them on a brand-name biologic for three years. Their skin was clear. Then their insurance forced a switch to a biosimilar. Within six weeks, the plaques returned. The patient lost work days. Their quality of life dropped. The doctor had to fight to get the original drug reinstated. That kind of story gets passed around among specialists.
It’s not anecdotal. A 2021 JAMA Network Open study showed that when prescribers or patients request brand-name drugs over generics, it adds $1.67 billion annually to Medicare costs. But behind that number are real people who got worse after a switch. Specialists aren’t ignoring cost-they’re weighing cost against risk.
The System Doesn’t Make Switching Easy
Even when a biosimilar is approved, it doesn’t mean it’s interchangeable. In the U.S., interchangeability is a legal status-not a clinical one. Only a handful of biosimilars have been officially designated as interchangeable by the FDA. That means pharmacists can’t automatically substitute them without the prescriber’s permission.
And when they do try to substitute? Patients get confused. Pharmacies get caught in the middle. Insurance companies push for savings, but specialists push back because they know what happens when patients miss doses or get confused about new regimens.
One rheumatologist in Toronto told a colleague: “I don’t care if the biosimilar is 80% cheaper. If my patient’s joints start swelling again because they switched and didn’t tell me, I’m the one who has to fix it.”
Specialists aren’t fighting change. They’re fighting chaos.
PBM Markups Are Making Generic Drugs More Expensive Than They Should Be
Here’s the twist: sometimes, the “cheaper” generic or biosimilar isn’t actually cheaper-at least not for the patient.
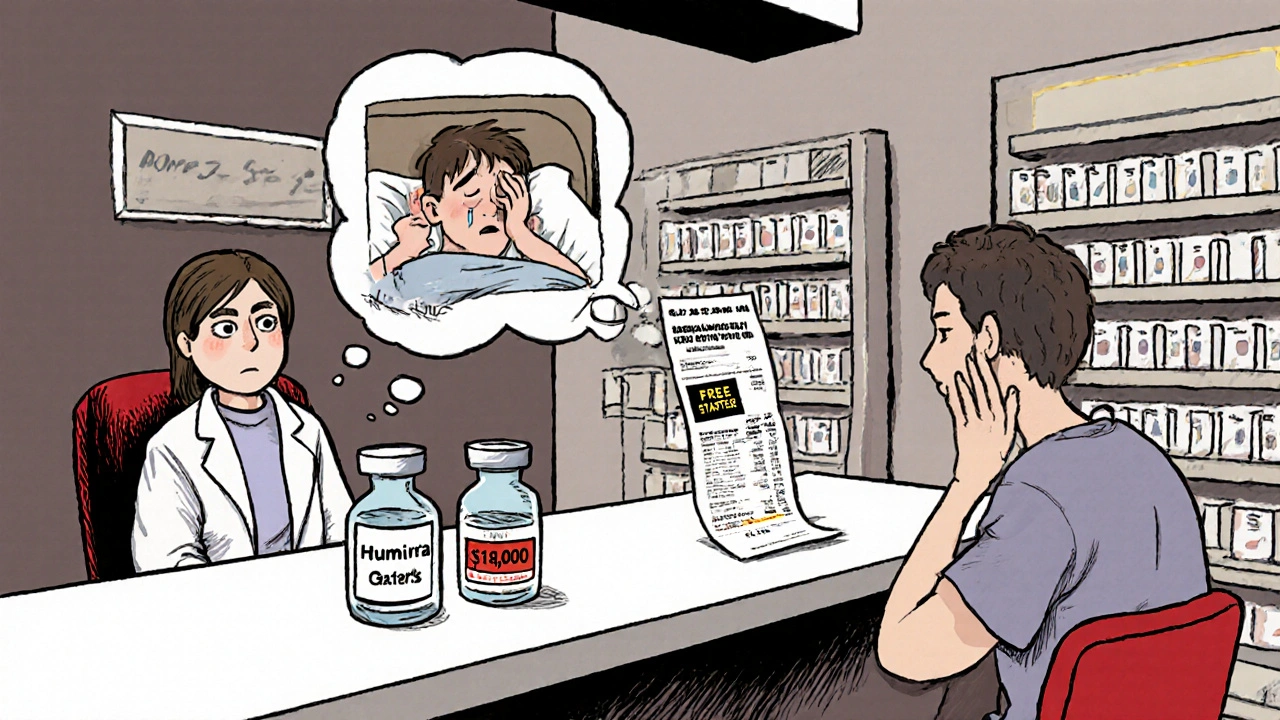
The Federal Trade Commission’s January 2025 report found that pharmacy benefit managers (PBMs)-the middlemen between insurers, pharmacies, and drugmakers-were marking up specialty generic drugs by thousands of percent. In some cases, a biosimilar that cost $5,000 to acquire was billed to the patient at $18,000.
Why? Because PBMs often own their own specialty pharmacies. When they control both the drug supply and the reimbursement system, they profit more from higher-priced drugs-even if those drugs are technically generics.
So when a specialist recommends a biosimilar, they’re not just fighting insurance-they’re fighting a broken pricing system. Sometimes, the brand-name drug has a patient assistance program. The biosimilar? No support. No copay card. No free starter kits. The patient ends up paying more out of pocket.
Dr. Peter Bach from Memorial Sloan Kettering put it bluntly: “The current system allows manufacturers to set prices without meaningful competition-especially for specialty drugs with limited alternatives.”
Patients Ask for the Brand-And Specialists Listen
It’s not just doctors pushing for brand-name drugs. Patients are asking for them.
On Reddit’s r/healthinsurance, users share stories like this: “My Humira copay went from $50 to $850 when my plan changed. My rheumatologist said biosimilars aren’t right for me. I don’t know why, but he’s the expert.”
Patients who’ve been on a brand-name drug for years know how it works for them. They’ve built routines around it. They’ve learned to manage side effects. They trust it. And when their insurance tries to force a switch, they push back-often with their doctor’s support.
Specialists aren’t just prescribing based on clinical guidelines. They’re responding to patient trust. And in chronic disease management, trust matters as much as data.
The Cost Isn’t Just Financial-It’s Emotional
For patients with rare or life-altering conditions, specialty drugs aren’t just medicine. They’re stability. They’re independence. They’re the difference between staying at home and being hospitalized.
One patient with multiple sclerosis described her Ocrevus infusions as “the only thing keeping me from using a wheelchair.” She didn’t care that the biosimilar was cheaper. She cared that her neurologist had seen hundreds of patients on this drug-and not one had a bad reaction.
That’s why specialists resist blanket policies that say “switch to biosimilar.” They’ve seen what happens when you treat patients like numbers in a spreadsheet. A 2024 study in the Journal of Managed Care & Specialty Pharmacy found that 42% of specialty drug starts are delayed by seven or more days due to administrative hurdles. For someone with progressive MS or aggressive cancer, that delay can mean irreversible damage.
It’s Not About Profit-It’s About Control
Some assume specialists prescribe brand-name drugs because they’re getting paid by drug companies. But the data doesn’t support that.
ProPublica’s 2016 analysis found that doctors who received over $5,000 from pharmaceutical companies prescribed brand-name drugs at a rate 50% higher than those who received nothing. That’s concerning-but it’s not the full picture.
Most specialists don’t take payments. They prescribe based on outcomes. And the outcomes they see? Brand-name drugs work consistently. Switching introduces variables they can’t control.
Plus, many brand-name manufacturers offer robust patient support: nursing hotlines, financial aid, home delivery, education materials. Biosimilar makers? Often none of that. A specialist doesn’t want to be the one who sends a patient home with a new drug and no one to call when they get dizzy, nauseated, or develop a rash.
What’s Changing? And What’s Not
The Inflation Reduction Act of 2022 lets Medicare negotiate prices for some high-cost drugs. That could eventually bring down prices for drugs like Jakafi, Ofev, and Xtandi. But negotiation takes time-and it doesn’t fix the underlying problems.
Until PBMs stop marking up generics by thousands of percent, until interchangeable biosimilars become the norm, and until patient support programs are equally available across all versions of a drug, specialists will keep prescribing the brand.
They’re not ignoring cost. They’re just prioritizing safety, consistency, and patient trust. And in specialty medicine, those things are worth more than a few dollars saved on a prescription.
What Patients Should Know
If your specialist recommends a brand-name specialty drug, don’t assume they’re being influenced by drug companies. Ask:
- Is there a biosimilar or generic available?
- Has it been proven to work the same way for my condition?
- Will I get the same support-nursing help, copay assistance, delivery-if I switch?
- What happens if I have a bad reaction after switching?
Many patients don’t realize they can ask for a prior authorization appeal. Or that manufacturer assistance programs exist-even for brand-name drugs. Don’t be afraid to push back on your insurer. Bring your specialist’s notes with you.
Specialists aren’t trying to drive up costs. They’re trying to keep patients stable. And in a system that’s broken in so many ways, that’s the only thing that matters.

Rusty Thomas
November 19, 2025 AT 20:34Dave Wooldridge
November 20, 2025 AT 16:24Rebecca Cosenza
November 22, 2025 AT 12:35swatantra kumar
November 23, 2025 AT 05:01Cinkoon Marketing
November 24, 2025 AT 09:53robert cardy solano
November 26, 2025 AT 09:21Pawan Jamwal
November 27, 2025 AT 13:49Bill Camp
November 28, 2025 AT 04:46Lemmy Coco
November 29, 2025 AT 09:40