When you think of a pharmacy, you might picture a busy corner store handing out pills for high blood pressure or antibiotics. But specialty pharmacy is a whole different world. These aren’t your everyday medications. They’re high-cost, complex drugs used for conditions like cancer, multiple sclerosis, rheumatoid arthritis, and hepatitis C. Many of them are biologics - proteins made from living cells - and they require refrigeration, special handling, and constant monitoring. And while generics have been a staple in community pharmacies for decades, bringing them into specialty pharmacy isn’t as simple as swapping a pill for a cheaper version. There are layers of complexity most people never see.
Why Specialty Drugs Are Different
Specialty medications often cost over $1,000 a month. Some, like certain cancer drugs or treatments for rare diseases, can run more than $100,000 a year. That’s why they’re not stocked on regular shelves. They’re managed through specialized pharmacies that handle everything from cold-chain shipping to patient education. These drugs usually come with strict protocols: patients need regular lab tests, injection training, and ongoing support to stay on track. A single missed dose or storage error can derail treatment.Unlike traditional prescriptions, where a pharmacist might automatically substitute a generic, specialty drugs often have no generic alternative. Many are biologics - large, complex molecules that can’t be exactly copied like small-molecule drugs. That’s why biosimilars are becoming so important. These aren’t generics, but they’re the closest thing we have. The FDA has approved over 35 biosimilars as of late 2023, with more on the way as big-name drugs like Humira lose patent protection.
The Generics Gap in Specialty Pharmacy
Most retail pharmacies dispense over 90% generics. But in specialty pharmacy, that number is often below 20%. Why? Because many specialty drugs simply don’t have generics. When they do, it’s usually years after the brand-name version hits the market. Take Copaxone, for example. The brand version cost about $78,000 a year for multiple sclerosis patients. When the generic glatiramer acetate arrived, the price dropped to around $45,000. That’s a huge win - but it took over a decade to get there.Even when generics are available, switching isn’t automatic. Many patients are stabilized on a specific brand. Switching them to a generic - even one approved by the FDA - can cause anxiety or even physical reactions. Patients report changes in side effects, fatigue, or mood after switching, especially with narrow therapeutic index (NTI) drugs like levothyroxine or warfarin. Pharmacists know this. They’ve seen patients panic when the pill color changes. The active ingredient is the same, but the fillers, dyes, or coating might be different. And those differences? They matter.
Supply Chain Chaos
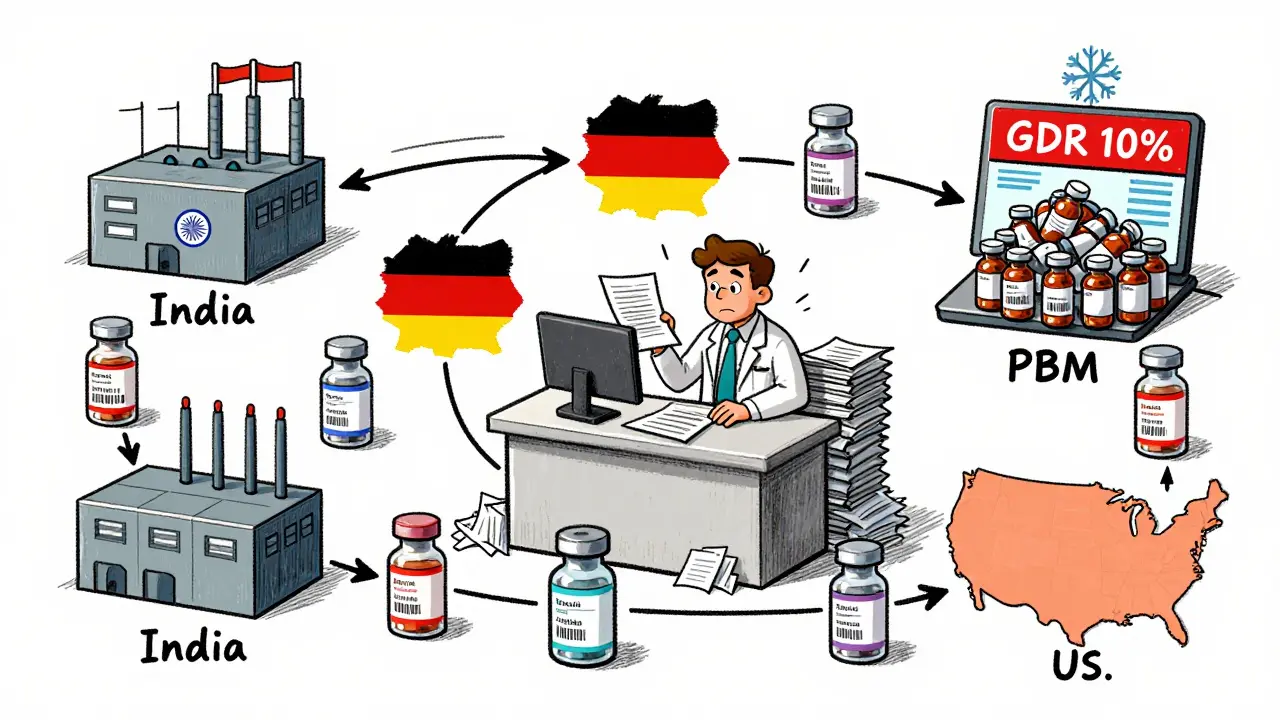
One of the biggest hidden challenges? The supply chain. Specialty pharmacies don’t just order from one distributor. They juggle multiple manufacturers for the same generic drug. One batch might come from India, another from Germany. Each has slightly different packaging, labeling, or even inactive ingredients. Tracking that? It’s a nightmare. A 2022 survey found that 65% of specialty pharmacy staff struggled with managing multiple manufacturers for the same product.And then there’s the reimbursement problem. Pharmacy Benefit Managers (PBMs) often reimburse specialty pharmacies below what they paid for the drug. That’s not sustainable. To make matters worse, PBMs track something called the Generic Dispensing Ratio (GDR). They expect specialty pharmacies to hit targets - even if there are no generics available for the drugs they’re dispensing. If you’re filling 100 prescriptions for a biologic with no generic version, and your GDR is 10%, you’re still penalized. It’s like being fined for not selling ice cream in a snowstorm.
When Generics Can Work - and When They Can’t
There are cases where generics make perfect sense. For example, some oral specialty drugs - like certain immunosuppressants or antivirals - now have generic versions. When a patient is stable, switching can save thousands. But it’s not a one-size-fits-all move. Pharmacists need to ask: Is this patient on a stable dose? Have they had any side effects in the past? Do they have allergies to certain fillers? Some patients are sensitive to lactose, dyes, or preservatives that vary between generic brands.For NTI drugs, even tiny changes in blood levels can cause problems. A study published in U.S. Pharmacist in 2022 recommended avoiding switches unless absolutely necessary. If a patient’s thyroid levels are perfectly balanced on one generic version, switching to another - even if it’s “bioequivalent” - might throw them off. That’s why documentation is key. Pharmacists need systems to flag patients who shouldn’t be switched. It’s not about being conservative. It’s about safety.
The Biosimilar Challenge
Biosimilars are the future - but they’re not generics. They’re not exact copies. They’re highly similar versions of biologic drugs, made from living cells. The FDA requires them to show no clinically meaningful differences, but the manufacturing process is so complex that minor variations are inevitable. That’s why substitution rules are different. In most states, a biosimilar can’t be automatically substituted like a generic. The prescriber must specifically order it.And PBMs are still figuring out how to handle them. Some place biosimilars on high-tier formularies, making patients pay more out of pocket. Others require prior authorization, even for drugs that have been on the market for years. That slows adoption. Meanwhile, patients hear “biosimilar” and think “cheaper, less effective.” Pharmacists spend hours explaining that Semglee (a biosimilar to Humalog) works the same way - but costs 40% less. Education isn’t optional. It’s part of the job.
What Works in Practice
Successful specialty pharmacies don’t just wait for generics to arrive. They plan for them. They start by identifying their top therapeutic areas - say, MS, rheumatoid arthritis, or hepatitis C - and align their generic sourcing with evidence-based guidelines. They standardize strengths and packaging to reduce confusion. They build relationships with one trusted distributor instead of juggling five. They train staff to recognize patient concerns before they escalate.They also track outcomes. After a switch, they don’t just assume it worked. They call patients. They check labs. They look for changes in adherence or side effects. One clinic in Toronto started following up with MS patients after switching to a generic glatiramer acetate. Within six months, they saw no drop in adherence - and a 40% reduction in patient out-of-pocket costs. That’s the kind of data that changes policy.
The Bottom Line
Specialty pharmacy isn’t about cutting costs. It’s about managing complexity. Generics and biosimilars offer real savings - but only if handled right. You can’t treat them like aspirin. You need systems. You need training. You need patience. And you need to listen to the patient who says, “This pill looks different. I don’t feel right.”The real value of specialty pharmacy isn’t in dispensing pills. It’s in holding the whole picture together - the drug, the patient, the supply chain, the insurance rules, and the fear behind the question: ‘Is this going to work like the old one?’”
Can generics be used for all specialty drugs?
No. Many specialty drugs, especially biologics used for conditions like cancer, MS, or rheumatoid arthritis, don’t have generic versions because they’re too complex to copy exactly. Only small-molecule specialty drugs - like some oral immunosuppressants or antivirals - have generics. Biosimilars are the closest alternative for biologics, but they’re not the same as traditional generics and require different handling.
Why do patients react differently to generic substitutions in specialty pharmacy?
Even though generics must meet FDA bioequivalence standards, they can have different inactive ingredients - like dyes, fillers, or coatings. These can affect how the drug is absorbed or how a patient feels. Some patients report fatigue, nausea, or mood changes after switching, especially with narrow therapeutic index drugs like levothyroxine or warfarin. The change in pill appearance can also cause anxiety, even if the drug works the same.
Are biosimilars the same as generics?
No. Generics are exact copies of small-molecule drugs. Biosimilars are highly similar to biologic drugs - which are made from living cells - but not identical. Manufacturing biologics is so complex that even small changes in the process can affect the final product. Biosimilars must prove they have no clinically meaningful differences, but they’re not interchangeable by default. Prescribers must specifically order them, and substitution rules vary by state.
Why do specialty pharmacies get penalized for low generic dispensing rates?
Pharmacy Benefit Managers (PBMs) track a metric called the Generic Dispensing Ratio (GDR) and set targets for pharmacies. But many specialty drugs have no generic version at all. If a pharmacy fills mostly biologics or complex therapies with no alternatives, they still get penalized for not hitting the GDR target. This creates unfair pressure and doesn’t reflect the reality of what specialty pharmacies actually dispense.
What should pharmacists do when a patient reports side effects after switching to a generic?
Don’t dismiss it. Document the change, review the patient’s medication history, and check if the new generic came from a different manufacturer. For narrow therapeutic index drugs, consider checking blood levels if appropriate. Talk to the prescriber - sometimes switching back is the safest option. Patient reports are valuable data points, not just complaints. Many studies show real variability in how patients respond to different generic versions, even when they’re FDA-approved.
How can specialty pharmacies improve their generic and biosimilar management?
Start by identifying your top therapeutic areas and aligning your generic sourcing with clinical guidelines. Use one trusted distributor to avoid supply chain chaos. Standardize packaging and strengths. Train staff to explain differences in appearance and manage patient concerns. Build protocols for monitoring patients after switches, especially for NTI drugs. And always document patient-specific contraindications - like allergies to certain excipients - in your system so they’re flagged every time.

Gloria Ricky
February 13, 2026 AT 04:41Ugh I just had to explain this to my mom last week. She switched my dad’s MS med to generic and he started zoning out like he was in a fog. I was like… mom, this isn’t aspirin. You can’t just swap pills and expect the same vibe. Pharmacist had to call the doc and switch it back. 😅
Sonja Stoces
February 13, 2026 AT 15:01LMAO so PBMs are penalizing pharmacies for not having generics for drugs that DON’T HAVE GENERICS?? 😂 Like bro, if you’re filling 90% biologics and your GDR is 12%, you’re getting fined for existing? This is the kind of bureaucratic nonsense that makes me wanna quit healthcare. 🤡
Rob Turner
February 14, 2026 AT 03:48It’s funny how we treat drugs like they’re all the same, but the body doesn’t care about FDA labels-it cares about how the pill *feels*. I’ve seen patients cry because the new generic was a different shade of blue. Not because it’s ‘less effective’-but because their body remembered the old one. There’s a psychological layer here we ignore at our peril. 🌿
Kristin Jarecki
February 16, 2026 AT 03:25While the emotional and logistical challenges surrounding generic substitution in specialty pharmacy are well-documented, it is imperative to emphasize that evidence-based protocols-when rigorously implemented-can mitigate risk. Standardized patient intake forms, electronic flags for NTI medications, and structured follow-up protocols have demonstrated statistically significant improvements in adherence and adverse event reporting. Institutional frameworks must prioritize patient-specific variables over blanket reimbursement metrics.
Jonathan Noe
February 17, 2026 AT 03:08Y’all are overcomplicating this. If the FDA says it’s bioequivalent, it’s bioequivalent. End of story. People are just scared of change. I worked in a specialty pharmacy for 8 years-switched 300+ patients to generics. Zero adverse events. Zero. You’re giving patients too much credit for being fragile. They just need to stop being drama queens. 🤷♂️
Jim Johnson
February 19, 2026 AT 01:42Bro I’ve been there. We had this one patient on Copaxone for 12 years. When the generic came out, we were all excited-until she called at 2 a.m. saying her hands felt like they were on fire. Turns out the new generic had a different dye. She’s allergic to FD&C Blue No. 1. We switched her back. No one told us about the dye. That’s the problem. We don’t track inactive ingredients like we should. This isn’t about fear-it’s about missing data. 🙏
christian jon
February 20, 2026 AT 05:41THEY’RE LYING TO US!!! 💥 The FDA doesn’t test generics the same way as brand names-EVERYONE KNOWS THIS! And PBMs? They’re just middlemen sucking blood from pharmacies and patients alike! I’ve seen pills with different fillers cause seizures-YES SEIZURES-and no one wants to admit it because ‘it’s bioequivalent’! Wake up, sheeple! This is Big Pharma’s dirty secret-manufacturers are CHANGING FORMULAS to cut costs and we’re the ones paying for it with our health! 🚨
Autumn Frankart
February 20, 2026 AT 10:20So if the government forces us to use generics but doesn’t fix the supply chain, who’s gonna get blamed when someone dies? China. India. The PBM. The pharmacist. The doctor. NO ONE. It’s a perfect storm of accountability avoidance. I’m not surprised. We’ve been sold a lie. 🇺🇸
Pat Mun
February 21, 2026 AT 02:05I love how this post dives into the human side of pharmacy-like, it’s not just about chemistry and cold-chain logistics, it’s about the woman who stares at her new pill and says, ‘This doesn’t feel like home.’ That’s real. That’s what matters. I work in a specialty pharmacy in rural Oregon, and we started doing weekly check-in calls after switches. It’s not in the budget, but we do it anyway. One guy said, ‘You’re the only one who asks if I’m okay.’ That’s why we do this. Not for the GDR. Not for the PBM. For them. 💙