Biosimilar Savings Calculator
How Much Could You Save?
Biosimilars typically cost 15-30% less than original biologic drugs. Calculate your potential savings.
Your Potential Savings
By switching to a biosimilar, you could save:
When you hear the word biosimilar, you might wonder: is this just a cheaper copy? Is it safe? Will it work the same? These aren’t just questions patients ask-they’re questions doctors, pharmacists, and insurers wrestle with every day. The truth is, biosimilars aren’t generics. They’re not simple chemical copies like the pills you pick up for high blood pressure. They’re complex, living-molecule drugs made from living cells, designed to match an already-approved biologic drug as closely as science allows. And after nearly two decades of real-world use, the evidence is clear: when approved by regulators like the FDA or EMA, biosimilars are just as safe and effective as the original biologics.
What Exactly Is a Biosimilar?
A biosimilar is a biological product that is highly similar to a reference biologic drug-like Humira, Enbrel, or Rituxan-that’s already been approved and used by millions of patients. It’s not an exact copy, because biological products are too complex to replicate perfectly. But the differences, if any, are minor and not clinically meaningful. That means they don’t change how the drug works in your body.
To get approved, a biosimilar must pass a mountain of testing. Regulators require detailed analysis of the molecule’s structure, purity, and how it behaves in the body. Then comes nonclinical testing-studies in cells and animals-and finally, clinical trials in humans. These trials compare how the biosimilar performs against the original drug in terms of safety, effectiveness, and side effects. The goal? To prove there’s no meaningful difference.
The FDA doesn’t require the same massive, multi-year trials that the original biologic went through. Why? Because the reference product’s safety profile is already well known. The biosimilar just needs to match it. That’s how they can be approved faster and still be just as reliable.
Are Biosimilars Safe? The Long-Term Data
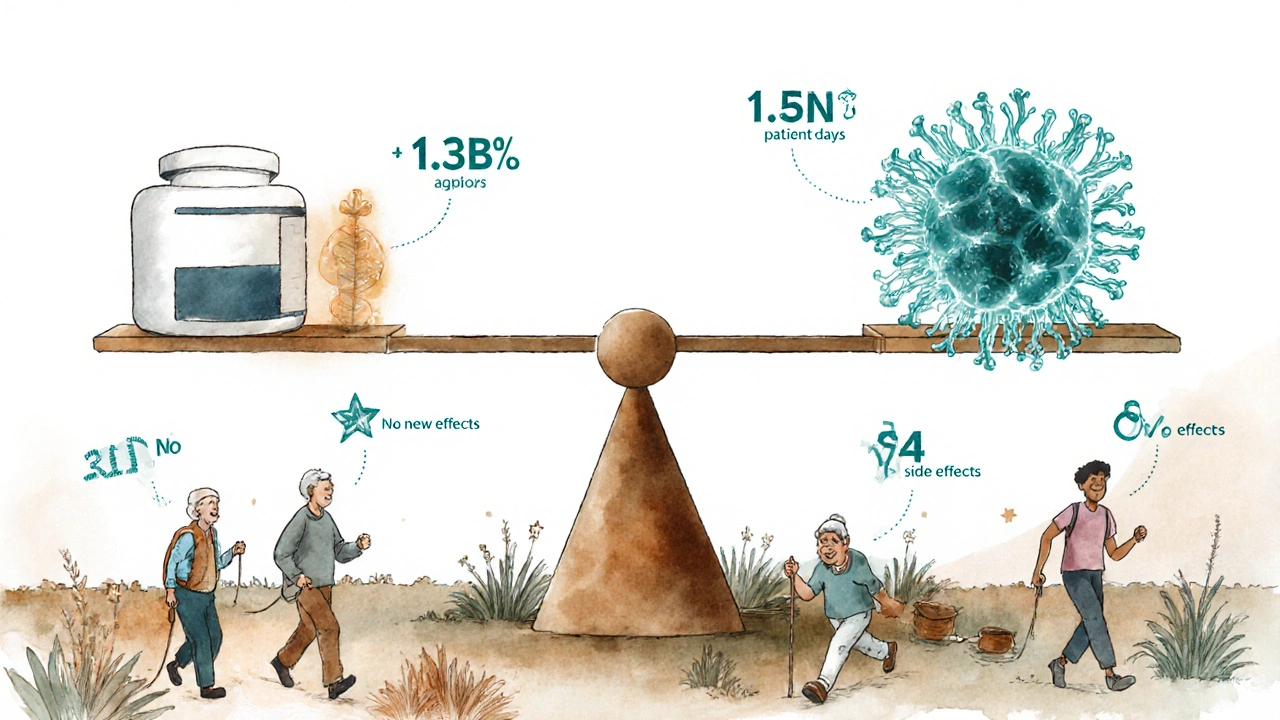
One of the biggest fears patients have is that switching to a biosimilar might cause unexpected side effects. But the numbers tell a different story. Sandoz, one of the leading biosimilar manufacturers, tracked over 1.3 billion patient treatment days across eight of its biosimilars-including drugs for arthritis, cancer, and immune disorders. That’s more than a billion days of real people using these medications in everyday life, not just in controlled trials.
What did they find? No new safety signals. No spike in serious side effects. No increase in immune reactions compared to the original drugs. Even for drugs like adalimumab (used for Crohn’s, psoriasis, and rheumatoid arthritis), where immunogenicity-the body making antibodies against the drug-is a known risk, long-term data shows biosimilars perform just as well.
And it’s not just one company. The European Medicines Agency (EMA), which approved the first biosimilar back in 2006, has collected data from over 55 approved biosimilars. The U.S. Food and Drug Administration (FDA) has approved 26 as of 2025, with 12 new ones approved in 2023 alone. Each one went through the same rigorous review. The FDA’s official stance? Biosimilars have no clinically meaningful differences in safety, purity, or potency from their reference products.
Do They Work as Well?
Effectiveness isn’t just about whether a drug lowers a lab value. It’s about whether patients feel better, stay in remission, avoid hospitalizations, and keep living their lives.
Studies like ClinicalTrials.gov’s NCT03729674 are designed to answer this directly. They track real outcomes: how long patients stay on treatment, whether their disease flares up, how many have serious side effects, and whether they need to stop the drug. Across multiple conditions-rheumatoid arthritis, inflammatory bowel disease, and certain cancers-the results are consistent. Biosimilars perform just like the originals.
One patient on MyBiosimilarsExperience.com switched from Humira to Amjevita (a biosimilar) because her insurance required it. After 18 months, she reported no difference in symptom control-and saved $1,200 a month. That’s not an outlier. A 2022 survey by the Biosimilars Council found that 68% of physicians reported positive outcomes with biosimilars. In Europe, biosimilars now make up 65% of the market for filgrastim (a drug used after chemotherapy) and 55% for infliximab. If they didn’t work, those numbers wouldn’t hold.
What About Switching? Is It Risky?
Many patients worry: if I’ve been on Humira for five years, is it safe to switch to a biosimilar? What if I switch back? The answer, backed by science and real-world use, is no.
Early concerns about switching were based on theory, not data. Now we have years of evidence. The FDA updated its guidance in February 2024 to say that switching between a reference product and a biosimilar-back and forth-is not associated with increased risk. This isn’t just opinion. It’s based on data from over a billion patient days.
Even more telling: studies on biosimilar-to-biosimilar switching (switching from one biosimilar to another) show no drop in effectiveness or rise in side effects. That’s important because in some countries, multiple biosimilars for the same drug are available. If switching between them was risky, we’d see a pattern of patients getting worse. We don’t.
One patient on a forum reported new rashes after switching to a biosimilar infliximab and felt better after switching back. That sounds alarming-but isolated anecdotes aren’t proof. Pharmacovigilance systems, which track side effects nationwide, show no increase in such reactions linked to biosimilars. The FDA and EMA monitor every reported adverse event. If there was a pattern, we’d know by now.
Why Aren’t More People Using Them?
If biosimilars are safe, effective, and cheaper, why is adoption still slow-especially in the U.S.?
Part of it is misinformation. Originator drug companies have spent millions marketing the idea that biosimilars are “highly similar, but not identical.” That sounds scary. But the FDA clarifies: “not identical” doesn’t mean “less safe.” It’s like saying two identical twins aren’t the same person-they’re not carbon copies, but they share the same DNA and behave the same way.
Patient awareness is low. A 2019 AMA Journal of Ethics report found many patients believed biosimilars were less effective or riskier-even though the evidence says otherwise. Doctors, too, sometimes hesitate, not because they doubt the science, but because they’re unsure how to explain it.
Then there’s the money. Biologic drugs are expensive, and manufacturers protect their profits with complex patent strategies and rebates to insurers. This creates a system where the cheapest option isn’t always the one pushed first. In Europe, where pricing is more regulated, biosimilars dominate. In the U.S., they’re used in only about 35% of cases for drugs like filgrastim.

How Do You Know If You’re Getting a Biosimilar?
Biosimilars have unique names. The FDA requires a four-letter suffix added to the generic name to distinguish them. For example, adalimumab is the original. Amjevita is adalimumab-atto. Cyltezo is adalimumab-adbm. This isn’t random-it’s to track side effects accurately.
Pharmacists can substitute an interchangeable biosimilar without asking your doctor, depending on your state’s laws. But if the biosimilar isn’t labeled as “interchangeable,” your doctor must specifically prescribe it. Always check your prescription label or ask your pharmacist if you’re unsure.
What’s Next for Biosimilars?
The market is exploding. The global biosimilar market was worth $9.3 billion in 2022. By 2030, it’s expected to hit $58.1 billion. That’s because more biologics are losing patent protection. Humira, the best-selling drug in history, now has four biosimilars approved in the U.S. alone.
Biosimilars are also moving into new areas. As of early 2024, 17 biosimilars are approved for cancer treatments. More are coming for diabetes, blood disorders, and rare diseases. The World Health Organization, FDA, and EMA all agree: biosimilars approved through their rigorous pathways are as safe and effective as the originals.
The biggest barrier now isn’t science-it’s perception. And that’s changing. As more patients and doctors see firsthand that a biosimilar works just as well, saves money, and doesn’t increase risk, adoption will grow. The data doesn’t lie. The experience doesn’t lie. The future of biologic treatment isn’t just about the original drug anymore-it’s about having choices that work, and work well, without breaking the bank.
Are biosimilars the same as generic drugs?
No. Generic drugs are exact chemical copies of small-molecule drugs like aspirin or metformin. Biosimilars are copies of large, complex biological drugs made from living cells. They’re highly similar, but not identical, because biological products are too intricate to replicate exactly. Still, they work the same way in the body and are equally safe and effective.
Can I switch from a biologic to a biosimilar safely?
Yes. Multiple studies, including long-term real-world data from over a billion patient treatment days, show no increased risk when switching from a reference biologic to a biosimilar-or back again. The FDA confirms that switching doesn’t affect safety or effectiveness. Many patients switch without any change in how they feel or any new side effects.
Why are biosimilars cheaper if they’re just as good?
Biosimilars cost less because manufacturers don’t have to repeat the massive, expensive clinical trials the original biologic went through. They only need to prove similarity to the already-approved drug. That cuts development time and cost. Savings are typically 15-30% lower than the original, and in some cases even more. Since 2015, biosimilars have saved the U.S. healthcare system over $31 billion.
Do biosimilars cause more side effects?
No. Regulatory agencies like the FDA and EMA require biosimilars to match the original drug’s safety profile. Long-term studies show no increase in side effects, including immune reactions. While any drug can cause side effects, biosimilars have not been linked to higher rates than their reference products.
How do I know if my doctor prescribed a biosimilar?
Check the prescription label. Biosimilars have a four-letter suffix added to the generic name, like adalimumab-atto (Amjevita) or infliximab-dyyb (Avsola). Your pharmacist will also let you know if you’re receiving a biosimilar. If you’re unsure, ask your doctor or pharmacist directly.

Stephen Adeyanju
November 25, 2025 AT 16:28james thomas
November 26, 2025 AT 02:10Deborah Williams
November 26, 2025 AT 08:01Micaela Yarman
November 26, 2025 AT 09:30Brittany Medley
November 27, 2025 AT 06:30Marissa Coratti
November 28, 2025 AT 20:27Ali Miller
November 29, 2025 AT 08:45Amanda Wong
November 30, 2025 AT 04:47Asia Roveda
November 30, 2025 AT 09:01mohit passi
December 1, 2025 AT 06:10Aaron Whong
December 1, 2025 AT 22:14Sanjay Menon
December 2, 2025 AT 14:42Rachel Whip
December 3, 2025 AT 04:30Ezequiel adrian
December 3, 2025 AT 18:06JAY OKE
December 5, 2025 AT 07:30